Abstract
Introduction Data comparing Lenalidomide with Thalidomide as combination partner of Carfilzomib are limited in multiple myeloma (MM). Carfilzomib maintenance therapy is efficacious in transplant-eligible pts, but results in transplant non-eligible (TNE) pts are not available. Here we compare KRd with KTd induction therapy and K maintenance treatment with control in TNE pts with MM and provide information on QoL during induction and maintenance therapy.
Patients and methods One hundred twenty two pts have been enrolled (ITT population). Median age was 75 yrs, ISS stage I/II/III: 29 (23.8%)/48 (39.3%)/45 (36.9%), ECOG stage 0/1: 64 (52.5%)/58 (47.5%). t(4;14) ± del17p was noted in 15 (16.3%) of 92 pts with results available. Pts were randomized to 9 cycles of KRd or KTd, and 107 pts received at least one full cycle. Carfilzomib (K) was started with 20mg/m2 at d 1 of cycle 1, and was continued with 27mg/m2 for the first 2 cycles (d 1+2, 8+9, 15+16 schedule); followed by K administration at 56mg/m2 once weekly for a 28 d cycle. Thalidomide 100mg/d (50mg in pts ≥75 yrs of age), d 1-28, or Revlimid 25mg/d, d 1-21. Dexamethasone 40mg (20mg in pts ≥75 yrs of age) once/week. After induction, pts with ≥SD were randomized to K maintenance (d 1 and 15) for 12 cycles or observation. MRD was assessed by NGF with a sensitivity of 10-6 in pts with ≥VGPR. Survival estimates were calculated according to KM and survival curves were compared with the log-rank test. PFS and OS results presented are given for the ITT population. Quality of life was assessed monthly for 21 months using the EORTC Q30 and the myeloma module QLQ-MY20. This trial is registered on clinicaltrials.gov (NCT02891811).
Results Median follow-up was 25.3 mos, 15 pts discontinued therapy within the first cycle due to pt (3) or investigator (1) decision, AE/toxicity (8), death or progressive disease (2) or other reasons (1). Overall response rate was 91.3% in the entire group with available data (n=115). Results for sCR, CR, VGPR, PR, and ORR for KRd and KTd were similar for both groups (7.3%/10.0%, 27.3%/33.3%, 38.2%/35.0%, 14.5/16.7%, 87.3%/95.0%, respectively). Minor response was noted in 4 (3.5%), SD in 5 (4.3%) and PD in 1 (0.8%) pts.
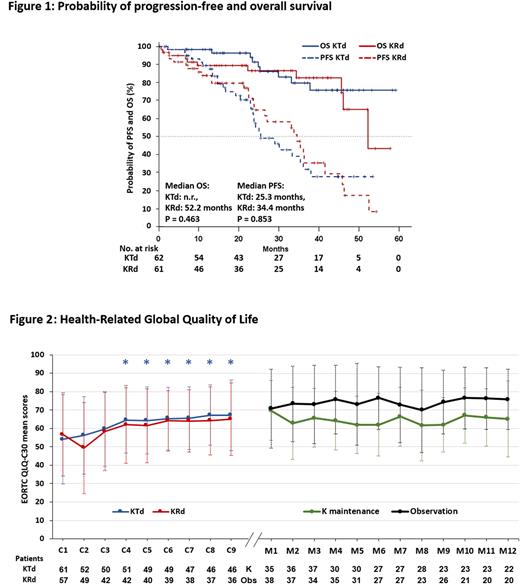
PFS (median 34.4 and 25.3 mos, p=0.853) and OS (52.2 mos vs not reached, p=0.463) were similar in the KRd and KTd group, respectively (Figure 1). The OS rate at 36 mos was 87% with KRd and 83% with KTd. MRD testing was performed in 60 pts at time of CR/VGPR. Of those, 48.3%, pts were found to be MRDneg (23.6% of the ITT population). PFS was significantly longer in MRDneg vs. MRDpos pts (p=0.002). Seventy-nine pts were randomized to K maintenance therapy or observation. Median PFS was numerically higher in pts with K maintenance treatment (median 25.2 vs 13.8, p=0.403), but the difference was not statistically significant. Data on OS after start of maintenance are not mature yet (only 9 events).
Baseline health-related global QoL improved numerically during induction therapy with KRd and clinically significantly at certain time points with KTd (by ≥10 score points, which is considered the threshold of clinical relevance (Figure 2)). Significant improvements have been obtained for physical functioning and pain with both regimens. Fatigue improved numerically with KRd and significantly with KTd. Neuropathy worsened significantly during induction therapy with KTd and at 2 time points only with KRd. During maintenance, KTd pre-exposed pts had significantly worse neuropathy scores. Carfilzomib maintenance therapy was associated with numerical and partly significant deterioration of health-related global QoL. Grade 3/4 hematologic AEs were anemia (4.1%), leukopenia (0.8%), thrombocytopenia (7.4%), while non-hematologic grade 3/4 AEs were infection (20.5%), GI-disorders (7.4%), hypertension (7.4%), renal and cardiac impairment/failure (6.6% and 8.2% respectively).
Conclusion Our data show similar efficacy of KRd and KTd in elderly NTE NDMM pts with no significant difference in ORR, PFS and OS. OS rate at 3 yrs was 87%/83% with KRd/KTd. Median PFS was significantly longer in MRDneg pts. PFS was numerically, but not statistically longer in pts on K maintenance vs observation. QoL data showed improvement in most dimensions of QoL, with the exception of higher neuropathy-scores in KTd-exposed pts and impairment of health-related global QoL during Carfilzomib maintenance therapy. Otherwise, treatment was associated with an acceptable tolerance profile.
Disclosures
Ludwig:abbvie: Honoraria; Janssen-Cilag: Consultancy, Honoraria, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GSK: Consultancy; Pfizer: Consultancy, Honoraria, Speakers Bureau. Melchardt:AbbVie: Honoraria; BMS: Honoraria. Zojer:Janssen: Honoraria; BMS/Celgene: Honoraria; Amgen: Honoraria; Sanofi: Honoraria; Takeda: Honoraria. Hartmann:BMS: Honoraria; Amgen: Honoraria; AbbVie: Honoraria; Janssen CIlag: Honoraria. Podar:Celgene: Consultancy, Honoraria; Amgen: Honoraria; Janssen: Consultancy, Honoraria; Takeda: Consultancy; Roche: Other: Research support. Willenbacher:BMS-Celgene: Consultancy, Honoraria, Other: Steering and Safety Committees, Research Funding; EUSA Pharma: Consultancy, Honoraria; syndena GmbH: Current Employment; Takeda: Consultancy, Honoraria, Research Funding; AMGEN: Consultancy, Honoraria, Other: Steering and Safety Committees, Research Funding; Abbvie: Honoraria; Gilead: Consultancy, Honoraria; Janssen-Cilag: Consultancy, Honoraria, Research Funding; GSK: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Kite: Consultancy; Novartis: Consultancy, Honoraria, Research Funding; Morphosys: Consultancy, Other: Safety and Steering Committees; Merck: Consultancy; Pfizer: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; Sandoz: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria, Research Funding; Fujimoto: Honoraria; Myelom- und Lymphomselbsthilfe Österreich: Honoraria; oncotyrol: Research Funding; DSMM: Other: Safety and Steering Committees. Schreder:AbbVie: Honoraria; Bristol-Myers Squibb: Honoraria; Janssen: Honoraria; Pfizer: Honoraria. Krauth:Takeda: Honoraria; Pfizer: Honoraria; Sanofi: Honoraria; GSK: Honoraria; BMS-Celgene: Honoraria; Janssen: Honoraria, Research Funding; AMGEN: Honoraria. Petzer:Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Sandoz: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite-Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Saegen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support. Schmitt:Astra Zeneca: Consultancy, Honoraria, Research Funding; BMS/Celgene: Consultancy, Honoraria; Janssen-Cilag: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria; abbvie: Consultancy, Honoraria. Machherndl-Spandl:Amgen: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria; Gilead: Consultancy, Honoraria. Agis:Janssen: Honoraria, Research Funding; AMGEN: Honoraria; BMS: Honoraria; Takeda: Honoraria. Knop:AMGEN: Honoraria; BMS: Honoraria, Other: Travel grant; Celgene: Honoraria; Sanofi: Honoraria; sobi: Other: Travel grant. Paiva:Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Adaptive: Honoraria; Roche Glycart AG: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; GSK: Honoraria, Research Funding; EngMab: Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Amgen: Honoraria; Gliead: Honoraria; Oncopeptides: Honoraria. Greil:Janssen-Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Astra Zeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; MSD Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; BMS-Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Hoffmann - La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal